Top Qs
Timeline
Chat
Perspective
Lisuride
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Lisuride, sold under the brand name Dopergin among others, is a monoaminergic medication of the ergoline family which is used in the treatment of Parkinson's disease, migraine, and high prolactin levels.[4][1] It is taken by mouth.[4][1]
Side effects of lisuride include nausea and vomiting, dizziness, headache, fatigue or drowsiness, insomnia or sleep, gastrointestinal disturbances such as abdominal pain or diarrhea, nasal congestion or runny nose, and hypotension, and hallucinations or confusion (particularly at higher doses).[4][5] Rarely, serious side effects such as cardiac or pulmonary fibrosis have been reported with long-term use, but they are extremely uncommon.[3]
Lisuride acts as a mixed agonist and antagonist of dopamine, serotonin, and adrenergic receptors.[4][1][6][7][8] Activation of specific dopamine receptors is thought to be responsible for its effectiveness in the treatment of Parkinson's disease and ability to suppress prolactin levels,[4][1] while interactions with serotonin receptors are thought to be principally involved in its effectiveness for migraine.[9][10] It is very similar in chemical structure to lysergic acid diethylamide (LSD).[4][5]
Remove ads
Medical uses
Lisuride is used to lower prolactin and, in low doses, to prevent migraine attacks.[1] The use of lisuride as initial antiparkinsonian medication for Parkinson's disease has been advocated, delaying the need for levodopa until lisuride becomes insufficient for controlling the parkinsonian symptoms.[1][additional citation(s) needed] Evidence is insufficient to support lisuride in the treatment of advanced Parkinson's disease as an alternative to levodopa or bromocriptine.[11][12]
Remove ads
Side effects
Side effects of lisuride include nausea and lowered blood pressure, among others.[3]
Pharmacology
Summarize
Perspective
Pharmacodynamics
Lisuride is a ligand of dopamine, serotonin, and adrenergic receptors as well as the histamine H1 receptor.[6] It has sub-nanomolar affinity for the dopamine D2, and D3 receptors, serotonin 5-HT1A and 5-HT1D receptors, and α2A-, α2B-, and α2C-adrenergic receptors, and low-nanomolar affinity for the dopamine D1, D4, and D5 receptors, serotonin 5-HT2A, 5-HT2B, and 5-HT2C receptors, α1A-, α1B-, and α1D-adrenergic receptors, and histamine H1 receptor.[6][21][22] Lisuride is a partial agonist of the D2, D3, D4, 5-HT2A, 5-HT2C, 5-HT5A, and H1 receptors, a full or near-full agonist of the 5-HT1A, 5-HT1B, and 5-HT1D receptors, and a silent antagonist of the 5-HT2B receptor and α1A-, α2A-, α2B-, and α2C-adrenergic receptors.[8][22][23][24][25] Due to its highly non-selective pharmacological activity, lisuride is described as a "dirty drug".[1] The effectiveness of lisuride in Parkinson's disease and hyperprolactinemia is thought to be mostly due to activation of dopamine D2 receptors.[1]
While lisuride has a similar receptor binding profile to the more well-known and chemically similar ergoline lysergic acid diethylamide (LSD; N,N-diethyllysergamide) and acts as a partial agonist of the serotonin 5-HT2A receptor likewise,[8] it lacks the psychedelic effects of LSD and hence is non-hallucinogenic.[26][1] Research suggests that the lack of psychedelic effects with lisuride may arise from biased agonism of the 5-HT2A receptor. Stimulation of the 5-HT2A protomer within the 5-HT2A–mGlu2 receptor complex evokes psychedelic effects, while these effects do not occur during sole stimulation of monomeric 5-HT2A receptors. Accordingly, different G proteins are involved.[27][28] Lisuride behaves as an agonist at the 5-HT2A receptor monomer. Since it competitively antagonizes the effects of LSD, it may be regarded as a protomer antagonist of the 5-HT2A–mGluR heteromer.[29] GPCR oligomers are discrete entities and usually possess properties distinct from their parent monomeric receptors. However, this theory is controversial, and other research has found that 5-HT2A–mGlu2 dimers may not be essential for psychedelic effects.[30][31] Lisuride shows weak or no Gq pathway recruitment and this may be responsible for its non-hallucinogenic nature.[18][32] Alternatively, lisuride is an extremely potent serotonin 5-HT1A receptor agonist, and this might inhibit serotonin 5-HT2A receptor-mediated hallucinogenic effects.[33]
Although lisuride has widely been said to be non-hallucinogenic, this may not actually be true.[5][34][35] Lisuride has been associated with incidence of visual and auditory hallucinations, sensory disturbances, delusions, and other hallucinogenic effects at high doses.[5][34][35] It may simply be that typical therapeutic doses of lisuride are too low to adequately engage the serotonin 5-HT2A receptor and produce hallucinogenic effects but that hallucinogenic effects can be produced at higher doses.[5] Both serotonin 5-HT2A receptor agonism and dopamine D2 receptor agonism might contribute to the hallucinogenic effects of lisuride.[5] Lisuride's potent activities at other receptors besides the serotonin 5-HT2A receptor and its associated prominent side effects at higher doses, like nausea, hypotension, blurred vision, and anxiety, may limit its potential for being dosed high enough to produce hallucinogenic effects.[5] In animals, lisuride partially to fully substitutes for LSD and other psychedelics in drug discrimination tests in rodents and monkeys, but does not produce the head-twitch response in rodents.[36][37][38][5][34][39][40] However, lisuride does produce the head-twitch response in the least shrew, a non-rodent species that is said to be highly sensitive to serotonin 5-HT2A receptor agonists.[41][42] When a modified drug discrimination paradigm is employed in which animals are trained to discriminate two training drugs (lisuride and LSD) and vehicle however, lisuride no longer substitutes for LSD.[37]
Lisuride dose-dependently suppresses prolactin levels due to its dopaminergic activity.[1][43] As an antagonist of the serotonin 5-HT2B receptor, lisuride has no risk of cardiac valvulopathy, in contrast to related ergolines like pergolide and cabergoline.[1]
Minute amounts of lisuride suppress the firing of dorsal raphe serotonergic neurons, presumably due to agonist activity at 5-HT1A receptors. [44] Noradrenergic neurons of the locus coeruleus were accelerated by the drug at somewhat higher doses, consistent with α1-adrenergic receptor antagonist activity. Pars compacta dopamine neurons demonstrated a variable response.
Pharmacokinetics
Absorption of lisuride from the gastrointestinal tract with oral administration is complete.[3] The absolute bioavailability of lisuride is 10 to 20% due to high first-pass metabolism.[3] The plasma protein binding of lisuride is 60 to 70%.[3] Peak levels of lisuride occur 60 to 80 minutes after ingestion with high variability between individuals.[3] The elimination half-life of lisuride is approximately 2 hours.[3] This is shorter than most other dopamine agonists.[3] Lisuride has more than 15 known metabolites.[3]
Remove ads
Chemistry

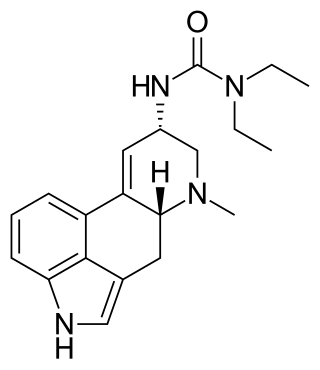
Lisuride, also known as 1,1-diethyl-3-(6-methyl-9,10-didehydroergolin-8α-yl)urea, is an ergoline derivative. It is almost identical in chemical structure to lysergic acid diethylamide (LSD), except that LSD's 8-position carboxamide group has been replaced with a urea group and the 8-position stereochemistry is inverted. Lisuride is described as the free base and as the hydrogen maleate salt.[45][46][47]
Analogues
Bromination of lisuride gives bromerguride (2-bromolisuride), which has a "reversed pharmacodynamic profile" compared to that of lisuride.[48]
Remove ads
History
Lisuride was synthesized by Zikán and Semonský at the Research Institute for Pharmacy and Biochemistry at Prague (later SPOFA) as an antimigraine agent analogous to methysergide and was described in 1960.[1][49] It was marketed by the early 1970s.[50]
Society and culture
Generic names
Lisuride is the INN and lysuride is the BAN.[45][51][46][47]
Brand names
Lisuride has been sold under brand names including Arolac, Cuvalit, Dopagon, Dopergin, Dopergine, Eunal, Lisenil, Lizenil, Lysenyl, Proclacam, Prolacam, and Revanil.[45][46][47][1]
Availability
Lisuride was previously more widely available throughout the world,[46][1] but as of 2020 it appears to be marketed only in Egypt, France, Italy, Kuwait, Lebanon, Mexico, New Zealand, and Pakistan.[47] Lisuride is not currently available in the United States, as the drug was not a commercial success.
Remove ads
Research
Preliminary clinical research suggests that transdermal administration of lisuride may be useful in the treatment of Parkinson's disease.[1] As lisuride has poor bioavailability when taken orally and has a short half-life, continuous transdermal administration offers significant advantages and could make the compound a much more consistent therapeutic agent.[1] Lisuride was under development as a transdermal patch and subcutaneous implant for the treatment of Parkinson's disease, restless legs syndrome, and dyskinesias in the 2000s and 2010s, but development was discontinued.[52][53]
Remove ads
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads